Carcinoma of unknown primary case reviewed in tumor board session
Karen Lusky
A molecular oncology tumor board session at CAP19 explored a case of cancer of unknown primary, presented by medical oncologist Alexander Drilon, MD, research director of early drug development at Memorial Sloan Kettering Cancer Center, and Rondell P. Graham, MBBS, head of GI/liver pathology at Mayo Clinic Rochester.
Dr. Drilon reported that the patient was 31 years old when she presented with progressive swelling in the left supraclavicular fossa. “Pertinent as part of her history, she had a previous diagnosis of stage IIIA Hodgkin lymphoma that was treated 16 years before she presented to us,” he said. When the medical records were scanned, they saw that she had been treated then with an ABVD regimen of chemotherapy and radiation to the abdomen and left supraclavicular fossa. Her past medical history was remarkable for hyperlipidemia and hypothyroidism. She had no prior surgeries. The physical exam “was notable only for a two-
Fig. 1
centimeter lymph node in the left supraclavicular fossa,” he said.
As part of their workup, the patient received a CT scan of the chest, abdomen, and pelvis and a PET scan, which demonstrated hypermetabolic lymphadenopathy in the bilateral supraclavicular regions, lower cervical chains, and mediastinum. “Her laboratory examinations were unremarkable, so a pretty bland workup except for a mild elevation of CA 19-9,” Dr. Drilon said. A local surgeon performed a left neck dissection.
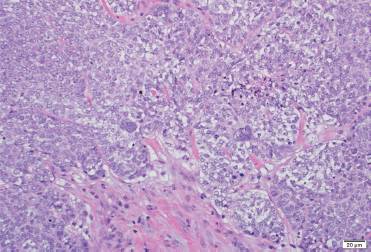
Dr. Graham displayed the image of a tissue specimen from a similar case (Fig. 1). “We have a neoplasm characterized by moderate-sized neoplastic cells with focal chromatin, prominent macronucleoli. And there are some occasional cells that show severe nuclear pleomorphism and multi-nucleation.” He also pointed out a brisk mitotic rate and some foci of necrosis. “A tumor that has a nonspecific look, such as this, is something we encounter rather frequently in our practice.”
The immunohistochemistry was positive for keratin 7 and negative for keratin 20, TTF-1, CDX2, thyroglobulin, vimentin, ER, PR, mammaglobin, GATA3, and PAX8. “So it was finally classified as a metastatic poorly differentiated carcinoma of unknown primary.”
Dr. Graham noted that 60 percent of carcinomas of unknown primary are adenocarcinomas. Thirty percent are poorly differentiated carcinomas (of an unknown type), and then there are small percentages of squamous cell carcinoma and neuroendocrine carcinoma, he said. There are therefore two broad categories of CUP: a common carcinoma type that pathologists can recognize (e.g. adenocarcinoma) and a high-grade/poorly differentiated carcinoma type they cannot specifically identify. “And even at this level, this dichotomy is useful to be able to include in your reports.”
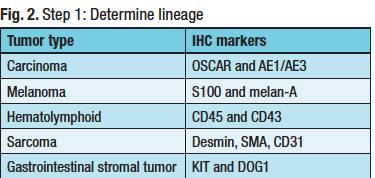
Dr. Graham described what he calls a “safe stepwise approach” to carcinoma of unknown primary workup. The first step: Determine the lineage. “It is a step that if one isn’t judicious, there is the potential for patient harm,” he cautions. As an example, “If the patient truly has a diagnosis of lymphoma, and it was instead signed out as a poorly differentiated carcinoma, there is the potential for patient harm because you may have an inappropriate treatment option offered to the patient,” he said in a CAP TODAY interview.
In step one (Fig. 2), Dr. Graham attempts to determine if the cancer is a carcinoma and uses two epithelial markers: OSCAR and AE1/AE3. He uses melan-A and S100 to test for melanoma, noting there are other markers. To detect a hematolymphoid cancer, he uses CD45 with CD43. Some lymphomas don’t express CD45, Dr. Graham explained, so he likes to use the combination. To screen for sarcomas, he chooses desmin, SMA, and CD31.
Dr. Graham also includes KIT and DOG1 for gastrointestinal stromal tumor in the initial panel because he has seen GIST overlooked on numerous occasions. “It is occasionally forgotten, and this is a diagnosis you can make and for which the patient can be treated.”
Narrowing the differential is step two (Fig. 3). Once Dr. Graham has completed the first step and suspects the cancer is a carcinoma, he finds keratin 7 and keratin 20 to be helpful. The markers are available in many practices, “and they narrow the differential diagnosis for carcinomas,” he said, “because there are some patterns where the differential diagnosis is really quite short.”
Dr. Graham uses synaptophysin and chromogranin to distinguish neuroendocrine neoplasms. If those markers are positive, he includes Ki-67. “The main value of Ki-67 is determining the tumor grade, which has prognostic value. It also informs the oncologist on its potential responsiveness to platinum-based chemotherapy in the context of neuroendocrine carcinoma,” he said.
“I do p40,” he added, “because if it is positive, I can look for whether it expresses high-risk HPV or not, because that narrows the differential diagnosis and may have therapeutic implications.”
If keratins 7 and 20 are negative, the pathologist has to contemplate a short list of four carcinomas: hepatocellular, prostate, renal cell, and adrenocortical. “If you see this profile, you have to rule out these differential diagnoses,” he said. A keratin 20 positive and keratin 7 negative also has a short differential: GI, urothelial cell carcinoma, and Merkel cell carcinoma. “By contrast, keratin 7 only positive carcinoma has a long differential. It can be difficult to remember all of the differential diagnoses at the top of your head, but once one has the keratin 7-20 profile in this context, the rest of the algorithm should efficiently lead to an answer.”
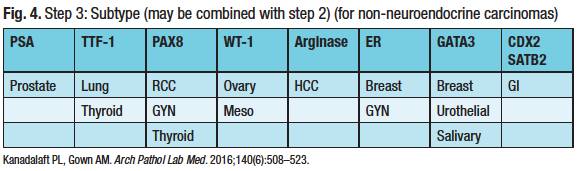
Step three is the refined search for the carcinoma subtype (Fig. 4) and may be concurrent with step two. “I do look at clinical information to see how to narrow and tailor this, if that is available to me,” Dr. Graham said. But his point is that “with a very limited pattern of markers, you can get to most diagnoses.”
Dr. Graham pointed out the caveats for step three. “For example, GATA3 can be positive in a lot of different tumor types. The initial papers touted its sensitivity for breast cancer and for urothelial carcinoma, but many different tumor types can be positive for GATA3.” TTF-1 is positive in neuroendocrine carcinomas, not only in the lung. “If someone has a small cell carcinoma, which is a histologic type of neuroendocrine carcinoma, regardless of the site of origin, TTF-1 can be positive.” One example is a small cell carcinoma from the urinary bladder.
A review of 12 studies found that the five most common occult primaries identified at autopsy are lung cancer, pancreatobiliary malignancy, other GI tumors, breast cancer, and ovarian carcinoma, Dr. Graham reported. “You want to make sure that when you finish your workup and you are ready to sign it out as a cancer of unknown primary, that you considered or excluded these possibilities as much as possible” (Massard C, et al. Nat Rev Clin Oncol. 2011;8[12]:701–710).
The primary malignancies “you don’t want to miss,” he said, “because they have favorable prognoses and good treatment options,” are as follows:
• Breast cancer. If a patient has axillary lymphadenopathy, it is reasonable to be worried about breast cancer, he said.
• Prostate cancer. Blastic bone metastases and an elevated serum PSA would be suggestive of prostate cancer. “There is a newer prostate marker, NKX3.1, which is excellent if you have it in your lab,” he said.
• Colorectal cancer. Colorectal endoscopic findings may be helpful for their negative predictive value.
• Head and neck squamous cell carcinoma. Cervical lymph nodes that are positive for carcinoma and for high-risk HPV strongly suggest oropharyngeal and base of tongue primaries, he said.
• Extragonadal germ cell tumor. “If you have mediastinal or retroperitoneal nodules or a mass in a young man, this is an important diagnosis to consider,” Dr. Graham said. In young adults, particularly men, he advises making sure that the prospect of a germ cell tumor has been ruled out.
Four IHC pitfalls are common sources of problems. No. 1 is skipping the first step; the pathologist does not determine the line of differentiation. No. 2 is lack of awareness of the “traps” in step three. For example, “urothelial primaries can show intestinal differentiation,” he explained, “and mucinous lung carcinoma can mimic a GI primary by immunohistochemistry.” The other two pitfalls: a failure to check the IHC controls and mislabeled slides.
To avoid exhausting the block before making a diagnosis, he offers these tips: split cores into separate blocks, face block for H&E and unstained at the same time, review H&E with clinical data before ordering IHC, and consider getting opinions before ordering.
Returning to the tumor board case, Dr. Drilon said their previous workup didn’t strongly suggest the tumor’s lineage. So the patient’s local physicians treated her with platinum-based doublet therapy. “It wasn’t clear why this particular regimen was chosen, although as per the local oncologist, the initial thought was this might have been a lung primary that manifested mostly as having metastases, with the occult lung primary not being found obviously on imaging. But thankfully, the patient had at least half a year of disease control, stable disease with this chemotherapy,” before there was progression in bone.
“Again, given that the local oncologist thought this might be more of a lung primary,” he continued, “pemetrexed was subsequently administered with five months of benefit, minor disease shrinkage. Unfortunately, that was again followed by worsening, widespread lymphadenopathy, and new metastases that involved the bone and new bilateral pulmonary nodules.”
To help further characterize the case, the patient’s initial tumor biopsy was sent for next-generation sequencing. “By this time, the patient had seen us at Memorial Sloan Kettering Cancer Center where we have an internal next-generation sequencing assay, MSK-IMPACT,” Dr. Drilon said, describing it as a broad hybrid-capture–based—not ampliconbased—NGS platform that interrogates more than 460 different genes for copy number changes, recurring gene fusions, single nucleotide variants, and hotspot substitutions.
Using the panel, they discovered an IRF2BP2-NTRK1 fusion. “This was definitely in-frame and looked like it was an activating event because it included the kinase domain,” Dr. Drilon said. No other colonical tumor drivers were found in the sample. A splice site TP53 X25_ mutation was the only other alteration detected. “Our assay comes with an assessment of mutation burden and microsatellite stability or instability. And this tumor was deemed to be microsatellite stable with a low tumor mutation burden, which we do tend to see with these cancers that are driven by a colonical driver.”
Dr. Graham spoke about the role of molecular profiling in CUP from the laboratory’s viewpoint, and in doing so he was “wearing two hats,” he said, as a GI anatomic pathologist and a molecular pathologist. “When I talk about molecular profiling in CUP in my AP role, most colleagues are thinking about commercially available molecular assays to assign a site of origin. These are assays that are based on gene expression profiling looking at miRNAs or mRNAs to assign a site of origin.”
He describes the assays as robust and supported by the literature, yet he doesn’t think they’re used extensively. Cost of testing and turnaround time are likely to be the two main reasons, he said. “A thoughtful panel of immunostains in many settings would typically be less expensive to do than commercial gene expression profiling tests. Turnaround time expectations may be more challenging with send-out testing.”
When he and colleagues in molecular pathology consider molecular profiling for diagnosis, they are “thinking about detecting fusion genes and specific DNA mutations that may be disease-defining,” he said. These sets of molecular assays are most useful for sarcomas, he noted, but beyond the scope of the case presented in the session.
“On the surface alone,” he cautions, there is a pitfall: “If you send a case for molecular profiling, you have to be cognizant of the morphology because the molecular result alone could lead you down the wrong path or lead to unresolved ambiguity.” As an example, the fusion EWSR1-CREB1 is found in angiomatoid fibrous histiocytoma, and in clear cell sarcoma of tendon sheath, which is an entirely different entity. “It’s also seen in clear cell sarcoma-like tumor of the GI tract, which is a highly aggressive malignancy. So it is prudent to be cautious of the use and interpretation of molecular testing without any clinical context or morphologic context.”
Dr. Drilon said they selected NTRK for the session because there are now two FDA-approved TRK inhibitorsentrectinib and larotrectinib—for patients with TRK fusion-positive cancers of any type. At MSK, if any driver alteration is identified matching someone to an FDA-approved therapy, or a clinical trial, an email is sent to the patient’s oncologist and to the principal investigator of that trial. That’s how the patient in their case was matched for therapy. (More on that later.)
NTRK fusions aren’t structurally much different from colonical fusions involving, for example, ALK, ROS1, or RET. NTRK1, 2, and 3 encode the receptor types in kinase as TrkA, B, or C. Therefore, any of these three genes in the 3' position, as long as there is an in-frame event that includes the kinase domain, would be viewed as an activating event.
Dr. Drilon said he tends to view NTRK fusions in two major groups. There are the rare cancers, where the frequency of TRK fusions, depending on the series one looks at, exceeds 90 percent. “And that means the identification of a TRK fusion is almost pathognomonic of these disease states.” These four main histotypes include mammary analogue secretory carcinoma, a salivary tumor that is morphologically similar to secretory breast carcinoma. “The last two histologies are congenital fibrosarcoma, which also shares some pathologic features under the microscope with congenital mesoblastic nephroma.”
The second major group of malignancies is more common and harbors TRK fusions at much lower frequencies: lung cancer, melanoma, sarcomas, and GI tumors. “For some of these, the frequencies are really low. For lung cancer, for example, we prospectively looked at our cases and found that a TRK fusion is found in about 0.2 percent of cases.”
NTRK fusions are actionable, so the assay the institution uses or sends out for must offer sufficient coverage for TRK, Dr. Drilon said. If resources are not an issue “and you are able to bill for next-generation sequencing, that would be the preferred method of identifying these cases.” The advantage of hybrid-capture NGS assays over amplicon-based testing is that “they are better poised to capture these events.”
Reverse transcription PCR is one alternative, he said, “but the pitfall is that you would need to know what you are looking for in the sequence of the 5' and 3' events in order to find it; you are not looking for any events that might not have been annotated in the past.”
FISH is another option, but there are three genes, so three FISH assays are needed, “which increases the amount of tissue you need,” Dr. Drilon said.
Immunohistochemistry has emerged as a practicable screening test for TRK fusions, he said. “Why? Similar to ALK fusions and ROS1 fusions, when you see a meaningful expression of either TrkA, B, or C in a tumor specimen, granted there are some lineages like smooth muscle and neuroendocrine where you might see basal expression of TRK outside of the cancer that might interfere with your results, but for all other histologies or primaries, if you have a positive IHC, there is a good likelihood that if you do follow-up testing with NGS, or another assay, you may find a TRK fusion.”
It’s sometimes thought that if a good DNA-based NGS test doesn’t find anything in a tumor sample, “there’s no driver in the cancer,” Dr. Drilon said. He and colleagues at MSK investigated to see if that was true (Benayed R, et al. Clin Cancer Res. 2019;25[15]:4712–4722). “We took lung adenocarcinomas that you know are enriched for actionable drivers. We took all cases that were deemed negative by MSK-IMPACT, and we ran those cases through targeted RNA sequencing,” using anchored multiplex PCR with the ArcherDx assay. MSK-IMPACT missed an actionable event in about 15 percent of the cases. The actionable fusions detected by targeted RNA sequencing included ALK, MET, NRG1, NTRK, RET, ROS1, among others. What will be needed moving into the future, Dr. Drilon predicts, is complementary DNA- and RNA-based NGS testing “to maximize finding these actionable drivers, including NTRK.”
There is no perfect assay, Pranil K. Chandra, DO, vice president and chief medical officer of genomic and clinical pathology services at PathGroup, Nashville, Tenn., said in a CAP TODAY interview. (He wasn’t a presenter or an attendee.) “DNA capture-based assays struggle with detecting fusion breakpoints within large intronic regions containing repetitive elements,” he says, “especially NTRK2 and NTRK3. RNAbased NGS takes advantage of natural molecular biologic processes where problematic intronic regions present in DNA have been removed by splicing.”
The RNA-based assays aren’t flawless either, Dr. Chandra says. “You need to have good RNA, and as we all know, RNA is relatively more susceptible to degradation. As a result, RNA quality can be highly variable and of poor quality, especially when extracted from formalin-fixed, paraffin-embedded tissues. This requires good internal controls to minimize the likelihood of false-negative results.”
More published data are needed, Dr. Chandra says, to underscore the advantages and drawbacks of various testing platforms, including DNAbased and RNA-based NGS and IHC. “Once we have a good understanding of sensitivity and specificity, we’ll be in a better position to optimize laboratory testing for best patient care.”
PathGroup, which provides laboratory services to about 95 hospitals and thousands of outpatient physician clients, uses Roche’s Ventana pan-TRK IHC assay as a first-line test in evaluating advanced solid tumors. They have integrated the IHC assay into all of their algorithmically driven advanced solid tumor molecular testing protocols, Dr. Chandra says. “Any positive [pan-TRK IHC] result is followed through with a more specific molecular test that looks for the presence of NTRK fusion. We use an RNA-based next-generation sequencing assay that looks for NTRK1, NTRK2, and NTRK3 fusion.”
The practice began using pan-TRK IHC testing late in the first half of 2019, and Dr. Chandra and colleagues have identified two patients with NTRK fusions. One patient had an advanced thyroid cancer, the other patient an advanced colon cancer.
In the patient case presented in the CAP19 tumor board session, because the NTRK fusion was found early, the patient was enrolled in a trial for a selective TRK inhibitor and had a complete response to the therapy. Subsequent imaging has confirmed this, Dr. Drilon said, “and she remains on therapy three years into the treatment. So a happy ending, a good outcome, for this patient.”
Dr. Drilon thinks it is useful to revisit the genomics of the tumor at resistance. If patients have on-target resistance mediated by several kinase domain mutations (TrkA: G595R, F589L, G667C, A608D; TrkB: G639R, F633L, G709C; TrkC: G623R, F617L, G696A), these patients may be eligible for second-generation TRK inhibitors currently in clinical trials, repotrectinib and LOXO-195. “In the face of off-target resistance, which we have also observed in some cases, these patients can be referred for histology-specific standard of care.” (Examples of potential mechanisms for off-target resistance are KRAS mutation, MET amplification, BRAF mutation, and IGF1R activation.) (Drilon A. Ann Oncol. 2019;30[suppl 8]:viii23–viii30.)
In closing, Dr. Graham said “There is always this underlying question that comes when you have a high-grade tumor and you are trying to figure out what it is. How much more specific can you get, and does it matter? We are practicing now in an era where it does matter,” thanks to agents that provide hope for prolonged survival.
Karen Lusky is a writer in Brentwood, Tenn.